
Committed to generations
NEW & NEXT






Pediatric
Specialty Company

Board member of
Association of Hellenic Enterprises of Baby Food and Specialised Nutrition, member of Specialised Nutrition Europe

ISO
Certified Company

Green Punkt
Certified Company
We are...you!
Our company was created with this principle
in mind... Because you do it better when you
know the problems from the inside, whether
that means as a company or as INDIVIDUAL!

We are...you!
Our company was created with this principle
in mind... Because you do it better when you
know the problems from the inside, whether
that means as a company or as INDIVIDUAL!
E. Kalofolias
Co-founder and Managing Director
A sphere and a cube. A circle and
a square. A coincidence or a true
and deeper meaning?
We do want to offer to you as much information as possible about how we do it and why we do it. Offer to you the deep knowledge of what does every product offer and why it offers what promises, how it works, why it works better and if you have any questions we are here to give you all the answers.
Because we want you to be confident that what you're using is the best possible solution for you and your family!

We do want to offer to you as much information as possible about how we do it and why we do it. Offer to you the deep knowledge of what does every product offer and why it offers what promises, how it works, why it works better and if you have any questions we are here to give you all the answers.
Because we want you to be confident that what you're using is the best possible solution for you and your family!



CONTACT
US
Address
Cube Pharma & Nutrition
54 Menandrou str.
10431 Athens
Greece
Contact info
t. +30 210 5240963
f. +30 210 5240754
info@cube-pharmaceuticals.gr

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
BioGaia Protectis®Family
10x probiotic chewable tablets with lemon flavor
The world’s 1st probiotic in chewable tablets (2000)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For gasrtrointestinal health in children and adults
Gastrointestinal disorders caused by Antibiotics/Diarrhoea/Constipation/Abdominal pain, Vomiting
FOR USE BY
Children from 3 years and adults
_________________________________________________
_________________________________________________
BioGaia Protectis® probiotic chewable tablets are a simple, natural, safe and easy way to restore and maintain a healthy microbial balance in the gastrointestinal tract, as well as to improve digestive health and function.
BioGaia Protectis® probiotic chewable tablets are a simple, natural, safe and easy way to restore and maintain a healthy microbial balance in the gastrointestinal tract, as well as to improve digestive health and function.
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms.
It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
α) Protects the probiotic strain that surrounds it from
β) In human body
USE
GASTROINTESTINAL DISORDERS CAUSED BY ANTIBIOTICS
(diarrhoea, abdominal pain, flatulence)
PREVENTION & TREATMENT
DOSAGE (prevention)
1 tablet once daily between antibiotics + for 3 days afterwards
DOSAGE (treatment)
1 tablet once after each antibiotics administration + for 3 days after
_________________________________________________
FUNCTIONAL ABDOMINAL PAIN
(i.e. when there are no pathological causes)
TREATMENT
DOSAGE
1 tablet daily for at least 1 month
_________________________________________________
CHRONIC CONSTIPATION
(i.e. symptoms that persist for >2 weeks)
TREATMENT
DOSAGE
1 tablet once daily
_________________________________________________
SUDDEN GASTROINTESTINAL DISORDERS
(diarrhoea, vomiting, flatulence, etc.)
TREATMENT
DOSAGE
2 tablets once daily
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogiaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

BioGaia Prodentis®
30x probiotic lozenges with apple flavor
The world’s 1st and most studied probiotic for oral health
Oral health for children and adults
Gingivitis / Caries / Periodontitis / Mycosis / Halitosis / Peri-implant mucositis / Peri-implantitis
FOR USE BY
Children from 4 years and adults
_________________________________________________
_________________________________________________
BioGaia Prodentis® probiotic lozenges are a simple, natural, safe and easy way to restore and maintain a healthy microbial balance in the oral cavity, to reduce the chances of tooth decay, to prevent or treat gingivitis and in general to reduce or treat soft tissue inflammation, which is responsible for many oral health problems.
They contain the clinically studied combination of two probiotic strains, L. reuteri Protectis® and L. reuteri ATCC PTA 5465. L. reuteri Protectis® (L. reuteri DSM 17938), BioGaia Sweden’s proprietary probiotic bacterium, originally isolated in breast milk, is the world’s most studied probiotic in infants and children and one of the three most studied in adults. Its safety and efficacy have been demonstrated in 217 clinical studies and 180 journal publications. (Last update: January 2020.)
L. reuteri Protectis® and L. reuteri ATCC PTA 5465 fully meet the requirements of the WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the three surviving strains. This was also confirmed in vivo, when ingestion from healthy people resulted in significant colonisation of the stomach, duodenum and ileum.
L. reuteri Protectis® colonizes the entire gastrointestinal tract, prevents the growth of harmful microorganisms, activates the immune system and improves gastrointestinal function.
In addition, it is the only probiotic that produces the antimicrobial substance reuterin against pathogenic microorganisms, so it has a unique anti-pathogenic effect, which is ideally complemented by L. reuteri ATCC PTA 5465, that offers a combination of its outstanding ability to provide its clinically proven anti-inflammatory action.
It contains the unique probiotic strains Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®) + Limosilactobacillus reuteri ATCC PTA 5465 (former name Lactobacillus reuteri ATCC PTA 5465).
The unique natural substance Limosi –a high molecular weight exopolysaccharide (EPS) biofilm– surrounding the probiotic strains L. reuteri Protectis® + L. reuteri ATCC PTA 6475 also acts as a prebiotic for the symbiotic flora.
This biofilm:
a) Protects the probiotic strain that surrounds it from
b) In human body
USE – 4–17 YEARS
CARIES
(4–6 YEARS)
PREVENTION
DOSAGE
2 lozenges daily for 1 month
CARIES
(6–12 YEARS)
PREVENTION
DOSAGE
1 lozenge daily for 1 month
PRIMARY CARIES
(12–17 YEARS)
TREATMENT
DOSAGE
2 lozenges daily for 3 months
USE – FROM 18 YEARS
GINGIVITIS
PREVENTION & TREATMENT
DOSAGE
1 lozenge daily
_________________________________________________
PREGNANCY GINGIVITIS
TREATMENT
DOSAGE
1 lozenge daily for 4–7 weeks
_________________________________________________
PERIODONTITIS
TREATMENT
DOSAGE
2 lozenges daily for 3 months
(after antibiotic/antiseptic treatment)
_________________________________________________
PERI-IMPLANT MUCOSITIS/PERI-IMPLANTITIS
PREVENTION & TREATMENT
DOSAGE (prevention)
1 lozenge daily before implantation
DOSAGE (treatment)
2 lozenges daily for at least 1 month
(after antibiotic/antiseptic treatment)
_________________________________________________
MYCOSIS
TREATMENT
DOSAGE
2 lozenges daily
_________________________________________________
HALITOSIS
(BAD BREATH)
PREVENTION & TREATMENT
DOSAGE
1 lozenges daily
NOTE
For everyday use. Brush your teeth daily. BioGaia Prodentis® should ideally be taken after brushing. If you are using an antiseptic/mouthwash, take BioGaia Prodentis® half an hour after a mild antiseptic and one hour after a strong antiseptic.
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogaiagreece
ww.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

Imunoglukan P4H®
30 capsules
The world’s 1st beta-glucan from the edible mushroom Pleurotus ostreatus
The world’s most studied beta-glucan in children
Side-chain beta-glucan with unique immunoregulatory properties
Clinically studied in children from 3 years and adults
Natural immunoregulatory action for children and adults
FOR USE BY
Children from 7 years and adults
The content of the capsule can be administered with water or food
_________________________________________________
_________________________________________________
Immunoregulation in children and adults, which is based on the immunoregulatory properties of the natural functional ingredient Pleuran (IMG®). In a simplified description of the function of our immune system, we could say that an underactive immune system is vulnerable to infections etc., while when it is overactive, it can lead to allergies, chronic inflammation etc. Imunoglukan P4H® is a simple, natural, safe and clinically proven way of regulating the function and restoring balance to our immune system, with the benefits of restoring or maintaining it to normal levels.
The clinically proven Imunoglukan P4H® range of immunoregulatory products contains the clinically studied and patented natural functional ingredient IMG® from Pleuran s.r.o. (parent company of Imunoglukan Ltd.).
IMG® is a biologically active polysaccharide, which was isolated for the 1st time from the edible mushroom Pleurotus ostreatus.
IMG® remains insoluble in the small intestine and is not absorbed. Its structure is unique (side-chain) and it is the one that gives its immunomodulatory properties, as the properties of beta-glucans depend entirely on their structure, which is different, depending on their origin.
The side-chain structure of IMG® is essential for its recognition by the immune-active cells of the Gut-associated lymphoid tissue (GALT) via specific receptors on Peyer’s plaques, such as dextin-1. In this way, natural immunity [involving monocytes, macrophages, natural killer (NK) cells and dendritic cells, as well as the associated peptides, cytokines, such as interferons and interleukins, that regulate the immune response] is initially activated. Subsequently, acquired immunity (involving B- and T-lymphocytes, as well as the associated cytokines, the lymphokines) is activated.
USE
PREVENTION
(Preparation, regulation and training of the immune system)
DOSAGE
1-2 capsules daily for at least 3 months
Suggested use for 6 months (October–March).
Ideally, it should be taken on an empty stomach in the evening before bedtime or in the morning
TREATMENT
(In acute phase – infections, stress, fatigue)
DOSAGE
2–3 capsules daily for at least 3–7 days
Ideally, it should be taken on an empty stomach in the evening before bedtime or in the morning
NOTE
Not to be taken/administered to transplant patients. Administration to immunocompromised persons may be made only with doctor’s advice and supervision
_________________________________________________
Pleuran s.r.o./Imunoglukan Ltd. (daughter company)
_________________________________________________
Pleuran s.r.o., a world leader in the research of beta-glucans for more than 25 years, was the first to isolate from the well-known edible mushroom Pleurotus ostreatus the beta-glucan, a polysaccharide-polymer of glucose with a structure that differentiates it from the beta-glucans of bacteria, cereals and moulds. The know-how of Pleuran s.r.o. led to the patented method of extracting the natural functional ingredient IMG®. The safety and efficacy of Imunoglukan P4H® have been proven to date* in 65 clinical studies in more than 10 research centers worldwide, it is already marketed in more than 30 countries and its properties make it a unique, natural, immunomodulatory product.
* Last update: January 2021
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.imunoglukan.com/gr
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

Imunoglukan P4H®
120ml syrup
The world’s 1st beta-glucan from the edible mushroom Pleurotus ostreatus
The world’s most studied beta-glucan in children
Side-chain beta-glucan with unique immunoregulatory properties
Clinically studied in children from 3 years and adults
Natural immunoregulatory action for children and adults
FOR USE BY
Children from 3 years and adults
_________________________________________________
_________________________________________________
Immunoregulation in children and adults, which is based on the immunoregulatory properties of the natural functional ingredient Pleuran (IMG®). In a simplified description of the function of our immune system, we could say that an underactive immune system is vulnerable to infections etc., while when it is overactive, it can lead to allergies, chronic inflammation etc. Imunoglukan P4H® is a simple, natural, safe and clinically proven way of regulating the function and restoring balance to our immune system, with the benefits of restoring or maintaining it to normal levels.
The clinically proven Imunoglukan P4H® range of immunoregulatory products contains the clinically studied and patented natural functional ingredient IMG® from Pleuran s.r.o. (parent company of Imunoglukan Ltd.).
IMG® is a biologically active polysaccharide, which was isolated for the 1st time from the edible mushroom Pleurotus ostreatus.
IMG® remains insoluble in the small intestine and is not absorbed. Its structure is unique (side-chain) and it is the one that gives its immunomodulatory properties, as the properties of beta-glucans depend entirely on their structure, which is different, depending on their origin.
The side-chain structure of IMG® is essential for its recognition by the immune-active cells of the Gut-associated lymphoid tissue (GALT) via specific receptors on Peyer’s plaques, such as dextin-1. In this way, natural immunity [involving monocytes, macrophages, natural killer (NK) cells and dendritic cells, as well as the associated peptides, cytokines, such as interferons and interleukins, that regulate the immune response] is initially activated. Subsequently, acquired immunity (involving B- and T-lymphocytes, as well as the associated cytokines, the lymphokines) is activated.
USE
The side-chain structure of IMG® is essential for its recognition by the immune-active cells of the Gut-associated lymphoid tissue (GALT) via specific receptors on Peyer’s plaques, such as dextin-1. In this way, natural immunity [involving monocytes, macrophages, natural killer (NK) cells and dendritic cells, as well as the associated peptides, cytokines, such as interferons and interleukins, that regulate the immune response] is initially activated. Subsequently, acquired immunity (involving B- and T-lymphocytes, as well as the associated cytokines, the lymphokines) is activated.
PREVENTION
(Preparation, regulation and training of the immune system)
DOSAGE
1 ml per 5 kg body weight daily for at least 3 months
Suggested use for 6 months (October–March).
Ideally, it should be taken on an empty stomach in the evening before bedtime or in the morning
TREATMENT
(In acute phase – infections, stress, fatigue)
DOSAGE
2 ml per 5 kg body weight daily for at least 3–7 days
Ideally, it should be taken on an empty stomach in the evening before bedtime or in the morning
NOTE
Not to be taken/administered to transplant patients. Administration to immunocompromised persons may be made only with doctor’s advice and supervision
_________________________________________________
Pleuran s.r.o. / Imunoglukan Ltd. (daughter company)
_________________________________________________
Pleuran s.r.o., a world leader in the research of beta-glucans for more than 25 years, was the first to isolate from the well-known edible mushroom Pleurotus ostreatus the beta-glucan, a polysaccharide-polymer of glucose with a structure that differentiates it from the beta-glucans of bacteria, cereals and moulds. The know-how of Pleuran s.r.o. led to the patented method of extracting the natural functional ingredient IMG®. The safety and efficacy of Imunoglukan P4H® have been proven to date* in 65 clinical studies in more than 10 research centers worldwide, it is already marketed in more than 30 countries and its properties make it a unique, natural, immunomodulatory product.
* Last update: January 2021
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.imunoglukan.com/gr
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

iovir® PLUS nasal spray
20 ml with Carragelose®, Kappa-Carrageenan + sorbitol
The world’s 1st spray with a broad spectrum against upper respiratory viruses + natural nasal decongestion
The only suitable from 1 year
The only with Carragelose®
Treatment of upper respiratory viral infections
Relief from viral symptoms (nasal congestion)
Hydration (strengthening the natural defence mechanism)
FOR USE BY
Children from 1 year and adults
_________________________________________________
_________________________________________________
In large clinical studies in children and adults it was proven that:
_________________________________________________
The clinically proven iovir® range of antiviral medical devices contains the clinically studied and patented natural functional ingredient Carragelose® from the company Marinomed Austria, which is derived from red algae. The iovir® range of medical devices offers a simple, natural, safe and easy way to prevent and adjunctively treat viral infections of the upper respiratory tract. iovir® is designed and developed on a patented technology platform. The platform is used to develop therapies targeting more than 200 different respiratory viral strains responsible for the common cold and flu. Το iovir PLUS® (nasal spray) contains the natural functional ingredients Carragelose® and Kappa-carrageenan, which are derived from red algae. It has the unique property of creating a protective barrier to common cold viruses and traps upper respiratory viruses indiscriminately and broadly, preventing them from adhering to the mucosa and thus preventing their multiplication and spread. It also forms a moisturising protective membrane. Due to the action of Carragelose® and Kappa-carrageenan ingredients, this membrane remains longer in the nasal mucosa and serves as a natural barrier against external influences. This physiological moisture membrane also supports the body’s natural defence mechanisms against common cold viruses. Finally, its natural hypertonic action causes the reduction of excess fluid from the nasal tissue, resulting in a reduction of swelling of the nasal mucosa, freeing up breathing.
USE (NATURAL PROPERTIES – MECHANICAL ACTION – VIRUS ENTRAPMENT)
TREATMENT
iovir® PLUS has the ability to encapsulate viruses, forming a cocoon around them. This prevents the interaction of the viruses with the cells and inhibits the process of their multiplication and spread in the body.
RELIEF – HYDRATION
The protective natural moisture film provided by iovir® PLUS, ®, combined with the reduction of the cause that triggers inflammation in the nasal mucosa, offer immediate and prolonged relief from symptoms, such as nasal congestion, sore throat, feeling of dryness, etc.
NATURAL DECONGESTION
iovir® PLUS contains sorbitol, which decongests the nose with a natural, osmotic mechanism and frees breathing.
DOSAGE (prevention/treatment)
1 spray per nostril many times daily or according to individual needs
_________________________________________________
Μarinomed Biotech AG
_________________________________________________
Marinomed Biotech AG is an Austrian biotechnology company. It was founded in 2006 as a spin-off company from the University of Vienna. It specializes in the development of innovative products in the field of infectious diseases based on its patented technology platforms. Marinomed has received several prestigious awards for its outstanding research and development activities. To date (5/2020), it holds 8 patents and has a commercial presence in more than 40 countries.
_________________________________________________
STERILE MEDICAL DEVICE
_________________________________________________
www.iovir.eu
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

iovir® nasal spray
20 ml with Carragelose® and Kappa-Carrageenan
The world’s 1st topical spray against upper respiratory viruses
The only with Carragelose®
The only suitable from 1 year
Protection from upper respiratory viruses
Treatment of upper respiratory viral infections
Relief from viral symptoms (runny nose/nasal itch)
Hydration (dryness of nasal mucosa/strengthening of natural defence mechanism)
FOR USE BY
Children from 1 year and adults
_________________________________________________
_________________________________________________
In large clinical studies in children and adults it was proven that:
_________________________________________________
The clinically proven iovir® range of antiviral medical devices contains the clinically studied and patented natural functional ingredient Carragelose® from the company Marinomed Austria, which is derived from red algae. The iovir® range of medical devices offers a simple, natural, safe and easy way to prevent and adjunctively treat viral infections of the upper respiratory tract. iovir® is designed and developed on a patented technology platform. The platform is used to develop therapies targeting more than 200 different respiratory viral strains responsible for the common cold and flu. iovir® (nasal spray) contains the natural functional ingredients Carragelose® and Kappa-carrageenan, which are derived from red algae. It has the unique property of creating a protective barrier to common cold viruses and traps upper respiratory viruses indiscriminately and broadly, preventing them from adhering to the mucosa and thus preventing their multiplication and spread. It also forms a moisturising protective membrane. Due to the action of Carragelose® and Kappa-carrageenan ingredients, this membrane remains longer in the nasal mucosa and serves as a natural barrier against external influences. This physiological moisture membrane also supports the body’s natural defence mechanisms against common cold viruses.
USE (NATURAL PROPERTIES – MECHANICAL ACTION – VIRUS ENTRAPMENT)
PROTECTION
iovir® creates a natural protective film of moisture in the mucous membrane of the nasal cavity, which acts against exogenous agents, preventing viruses from adhering to the cell membrane receptors and thus naturally supporting the body’s defence.
TREATMENT
iovir® has the ability to encapsulate viruses, forming a cocoon around them. This prevents the interaction of the viruses with the cells and inhibits the process of their multiplication and spread in the body.
RELIEF – HYDRATION
The protective natural moisture film provided by iovir®, combined with the reduction of the cause that triggers inflammation in the nasal mucosa, offer immediate and prolonged relief from symptoms, such as runny nose, dryness, etc.
DOSAGE (prevention/treatment)
1 spray per nostril at least 3 times daily or according to individual needs
_________________________________________________
Μarinomed Biotech AG
_________________________________________________
Marinomed Biotech AG is an Austrian biotechnology company. It was founded in 2006 as a spin-off company from the University of Vienna. It specializes in the development of innovative products in the field of infectious diseases based on its patented technology platforms. Marinomed has received several prestigious awards for its outstanding research and development activities. To date (5/2020), it holds 8 patents and has a commercial presence in more than 40 countries.
_________________________________________________
STERILE MEDICAL DEVICE
________________________________________________
www.iovir.eu
ww.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

iovir® throat spray
20 ml with Carragelose® cherry flavour
The world’s 1st topical spray against upper respiratory viruses
The only with Carragelose®
The only suitable from 1 year
Protection from upper respiratory viruses
Treatment of upper respiratory viral infections
Relief from viral symptoms (sore throat/cough)
Hydration (hoarseness/feeling of dryness of the oropharyngeal mucosa)
FOR USE BY
For children from 1 year and adults
_________________________________________________
In large clinical studies in children and adults it was proven that:
_________________________________________________
The clinically proven iovir® range of antiviral medical devices contains the clinically studied and patented natural functional ingredient Carragelose® from the company Marinomed Austria, which is derived from red algae. The iovir® range of medical devices offers a simple, natural, safe and easy way to prevent and adjunctively treat viral infections of the upper respiratory tract. iovir® is designed and developed on a patented technology platform. The platform is used to develop therapies targeting more than 200 different respiratory viral strains responsible for the common cold and flu. iovir® (nasal spray) contains the natural functional ingredients Carragelose® and Kappa-carrageenan, which are derived from red algae. It has the unique property of creating a protective barrier to common cold viruses and traps upper respiratory viruses indiscriminately and broadly, preventing them from adhering to the mucosa and thus preventing their multiplication and spread. It also forms a moisturising protective membrane. Due to the action of Carragelose® and Kappa-carrageenan ingredients, this membrane remains longer in the nasal mucosa and serves as a natural barrier against external influences. This physiological moisture membrane also supports the body’s natural defence mechanisms against viruses of the upper respiratory tract.
USE (NATURAL PROPERTIES – MECHANICAL ACTION – VIRUS ENTRAPMENT)
PROTECTION
iovir® creates a natural protective film of moisture in the mucous membrane of the nasal cavity, which acts against exogenous agents, preventing viruses from adhering to the cell membrane receptors and thus naturally supporting the body’s defence.
TREATMENT
iovir® has the ability to encapsulate viruses, forming a cocoon around them. This prevents the interaction of the viruses with the cells and inhibits the process of their multiplication and spread in the body.
RELIEF – HYDRATION
The protective natural moisture film provided by iovir®, combined with the reduction of the cause that triggers inflammation in the nasal mucosa, offer immediate and prolonged relief from symptoms, such as cough, sore throat, hoarseness, feeling of dryness, etc.
DOSAGE (prevention/treatment)
2–3 sprays per nostril at least 3 times daily or according to individual needs
_________________________________________________
Μarinomed Biotech AG
_________________________________________________
Marinomed Biotech AG is an Austrian biotechnology company. It was founded in 2006 as a spin-off company from the University of Vienna. It specializes in the development of innovative products in the field of infectious diseases based on its patented technology platforms. Marinomed has received several prestigious awards for its outstanding research and development activities. To date (5/2020), it holds 8 patents and has a commercial presence in more than 40 countries.
_________________________________________________
STERILE MEDICAL DEVICE
_________________________________________________
www.iovir.eu
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

Capricare® 3 Powedered milk
400gr
The Original (1988) and No1 goat milk infant formula worldwide
Based on WHOLE goat milk
The only goat milk for infants evaluated by EFSA
FOR USE BY
Toddlers from 12 months and onwards
_________________________________________________
_________________________________________________
Milk powder for the nutritional coverage of infants after the 12th month.
It is prepared with whole goat milk as raw material instead of mixing ingredients, resulting in the mildest possible processing.
It complies with the European Infant Formula Directive and it is the only one clinically evaluated by the European Food Safety Agency (EFSA).
It is produced by the world leader in research, development and production of whole goat milk for infants, Dairy Goat Co-operative (DGC) New Zealand.
DGC’s unique and vertically integrated production process (breeding – milking – milk powder production – manufacturing of packaging materials – canning) ensures:
FASTEST PROCESS POSSIBLE
From milking the goats to milk powder within couple of days. It ensures the freshness and natural properties of whole goat milk.
LESS HARM TO THE RAW MATERIAL
The mildest heat treatment, with the least possible “disturbance” of the proteins and the lowest percentage of glycosylated compounds in the final product.
REDUCED RISK OF CONTAMINATION
No long-term storage of ingredients, large number of suppliers, transport and processing in other factories or countries.
QUALITY ASSURANCE
30 years of expertise. 100% focus on exclusive production of infant milk from whole goat milk. Total control of raw materials, packaging materials and all stages of production, ensuring the quality of the final product.
RESULT
The benefits of the unique natural properties of whole goat milk are retained in the final product:
_________________________________________________
Dairy Goat Co-operative (DGC)
_________________________________________________
Dairy Goat Co-operative (DGC) Ltd., Nea Zealand, is the world leader in infant milk from goat milk. DGC is the inventor of infant milk from goat milk (1988). Throughout these 30 years, DGC has managed to keep a continuous evolution and to develop a highly sophisticated model of expertise around infant milk based on goat milk, that lead to the change of the European legislation for infant milks in 2012. DGC is a highly integrated co-operation, where every step in infant milk production, from farming and milking to infant milk in the can, is handled, controlled and completed in whole by DGC. The final product is produced and packaged in the world’s most sophisticated production facility for infant milk, designed to produce exclusively infant milk from whole goat milk.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.capricare.gr
www.facebook.com/Capricare.Greece
www.instagram.com/capricaregreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

BioGaia Gastrus®
30x probiotic chewable tablets with mandarin flavor
Specially designed for problems related to the stomach
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For stomach health of children and adults
Gastritis / Helicobacter Pylori / Stomach disorders
FOR USE BY
Children from 3 years and adults
_________________________________________________
_________________________________________________
BioGaia Gastrus® probiotic chewable tablets are a simple, natural, safe and easy way to restore and maintain a healthy microbial balance in the gastrointestinal tract, to reduce inflammation and to treat functional gastrointestinal disorders caused during the eradication treatment of Helicobacter pylori.
BioGaia Gastrus® probiotic chewable tablets are a simple, natural, safe and easy way to restore and maintain a healthy microbial balance in the gastrointestinal tract, to reduce inflammation and to treat functional gastrointestinal disorders caused during the eradication treatment of Helicobacter pylori.
L. reuteri Protectis® and L. reuteri ATCC PTA 6475 fully meet the requirements set by the WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the three surviving strains. This was also confirmed in vivo, when ingestion from healthy people resulted in significant colonisation of the stomach, duodenum and ileum.
L. reuteri Protectis® colonizes the entire gastrointestinal tract, prevents the growth of harmful microorganisms, activates the immune system and improves gastrointestinal function.
In addition, it is the only probiotic that produces the antimicrobial substance reuterin against pathogenic microorganisms, so it has a unique anti-pathogenic action, which is ideally complemented by L. reuteri ATCC PTA 6475, which offers a combination of its outstanding ability to survive in the hostile environment of the stomach for more than 3 hours and provide its clinically proven anti-inflammatory action.
It contains the unique probiotic strains Limosilactobacillus reuteri ATCC PTA 6475
(former name Lactobacillus reuteri Protectis® ) + Limosilactobacillus reuteri ATCC PTA 6475
The unique natural substance Limosi –a high molecular weight exopolysaccharide (EPS) biofilm– surrounding the probiotic strains L. reuteri Protectis® + L. reuteri ATCC PTA 6475 also acts as a prebiotic for the symbiotic microflora.
This biofilm:
a) Protects the probiotic strain that surrounds it from
b) In human body
USE
GASTRITIS
PREVENTION & TREATMENT
DOSAGE
1 tablet daily
_________________________________________________
ERADICATION OF HELICOBACTER PYLORI & GASTRINTESTINAL ADVERSE EFFECTS FROM THE USE OF ANTIBIOTICS
(diarrhea, abdominal pain, flatulence)
PREVENTION & TREATMENT
DOSAGE
1 tablet daily during the eradication treatment
1 tablet daily for 2–3 weeks after eradication treatment
_________________________________________________
STOMACH DISORDERS
(indigestion, bloating, abdominal pain, stomach pains, reductions, etc.)
TREATMENT
DOSAGE
1–2 tablets daily or based on individual needs
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

BioGaia Protectis® ORS Family (orange flavour)
7 sachets for probiotic Oral Rehydration Solution + zinc, with orange flavor
The world’s 1st hydration solution with probiotic and zinc (2009)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For intestinal health in children and adults
Gastrenteritis / Dehydration / Diarrhea / Vomiting
FOR USE BY
Infants from 1 year, children and adults
_________________________________________________
_________________________________________________
It contains electrolytes, probiotic L. reuteri Protectis® and zinc. Available in a package of 7 sachets of 5.5 g. The contents of each sachet are dissolved in 250 ml of cool water, kept 3 hours out of the refrigerator or 6 hours in the refrigerator, and provides the following:
* WHO: World Health Organization
* ESPGHAN: European Society of Paediatric Gastroenterology, Hepatology and Nutrition
* ESPID: European Society for Paediatric Infectious Diseases
_________________________________________________
BioGaia Protectis® ORS, probiotic oral hydration solution + zinc, is a simple, natural, safe and easy way to prevent and treat dehydration, e.g. caused by gastroenteritis, as well as to restore and maintain a healthy microbial balance in the gastrointestinal tract, in order to reduce the intensity and duration of symptoms associated with gastroenteritis.
It contains L. reuteri Protectis® (L. reuteri DSM 17938), the patented probiotic bacterium of BioGaia Sweden. Originally isolated in breast milk, L. reuteri Protectis® is the world’s most studied probiotic in infants and children and one of the three most studied in adults. Its safety and efficacy have been demonstrated in 217 clinical studies and 180 journal publications (last update: January 2020).
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms, such as rotaviruses.
It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
a) Protects the probiotic strain that surrounds it from
b) In human body
Its action has been shown in large published clinical studies to provide:
_________________________________________________
+ ZINC
_________________________________________________
According to studies and observations by the WHO and UNICEF:
_________________________________________________
ELECTROLYTE COMPOSITION
_________________________________________________
The electrolyte composition of BioGaia Protectis® ORS is following the latest recommendations of WHO, UNICEF, ESPGHAN, ESPID.
According to the most recent studies and observations, the new formulation offers superior clinical benefits, and more specifically:
USE
ELECTROLYTIC DISORDERS AND DEHYDRATATION CAUSED BY GASTRENTERITIS (mild and moderate diarrhoea and/or vomiting), EPHIDROSIS, FEVER OR STRENUOUS EXERCISE, ETC.
PREVENTION & TREATMENT
DOSAGE
1–4 sachets daily, depending on age and needs.
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

BioGaia Protectis® + D3 Family
30x probiotic chewable tablets with orange flavor
The world’s 1st probiotic in chewable tablets (2000)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For gasrtrointestinal health in children and adults
Gastrointestinal disorders caused by Antibiotics / Diarrhea / Constipation / Abdominal pain / Vomit
With Vitamin D3: Proper immune function / Bone and dental health / Prevention of rickets
FOR USE BY
Children from 3 years and adults
_________________________________________________
_________________________________________________
BioGaia Protectis® probiotic chewable tablets are a simple, natural, safe and easy way to restore and maintain a healthy microbial balance in the gastrointestinal tract, as well as to improve digestive health and function.
BioGaia Protectis® drops contain L. reuteri Protectis® (L. reuteri DSM 17938), the patented probiotic bacterium of BioGaia Sweden. Originally isolated in breast milk, L. reuteri Protectis® is the world’s most studied probiotic in infants and children and one of the three most studied in adults. Its safety and efficacy have been demonstrated in 217 clinical studies and 180 journal publications (last update: January 2020).
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
L. reuteri Protectis® colonizes the entire gastrointestinal tract, prevents the growth of harmful microorganisms, activates the immune system and improves gastrointestinal function.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms.
+VITAMIN D
The recommended dosage of 1 tablet per day provides 800 IU of vitamin D according to the European Food Safety Authority (EFSA) for children and adults. A daily intake of 800IU of vitamin D is recommended to meet dietary vitamin D needs for proper bone development and dental health in children and the prevention of rickets. Meeting these needs is often difficult due to the dependence of intake on each person’s diet, sun exposure, skin color and underlying diseases.
L. reuteri Protectis® has been shown to improve the body’s absorption of vitamin D and works synergistically to strengthen the immune system. It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
a) Protects the probiotic strain that surrounds it from
b) In human body
USE
GASTROINTESTINAL DISORDERS CAUSED BY ANTIBIOTICS
(diarrhoea, abdominal pain, flatulence)
PREVENTION & TREATMENT
DOSAGE (prevention)
1 tablet once daily between antibiotics + for 3 days afterwards
DOSAGE (treatment)
1 tablet once after each antibiotics administration + for 3 days after
_________________________________________________
FUNCTIONAL ABDOMINAL PAIN
(i.e. when there are no pathological causes)
TREATMENT
DOSAGE
1 tablet daily for at least 1 month
_________________________________________________
CHRONIC CONSTIPATION
(i.e. symptoms that persist for >2 weeks)
TREATMENT
DOSAGE
1 tablet once daily
_________________________________________________
SUDDEN GASTROINTESTINAL DISORDERS
(diarrhoea, vomiting, flatulence, etc.)
TREATMENT
DOSAGE
2 tablets once daily
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

BioGaia Protectis® Family
60x probiotic chewable tablets with lemon flavor
The world’s 1st probiotic in chewable tablets (2000)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For gasrtrointestinal health in children and adults
Gastrointestinal disorders caused by Antibiotics / Diarrhea / Constipation / Abdominal pain / Vomiting
FOR USE BY
Children from 3 years and adults
_________________________________________________
_________________________________________________
BioGaia Protectis® probiotic chewable tablets are a simple, natural, safe and easy way to restore and maintain a healthy microbial balance in the gastrointestinal tract, as well as to improve digestive health and function.
BioGaia Protectis® drops contain L. reuteri Protectis® (L. reuteri DSM 17938), the patented probiotic bacterium of BioGaia Sweden. Originally isolated in breast milk, L. reuteri Protectis® is the world’s most studied probiotic in infants and children and one of the three most studied in adults. Its safety and efficacy have been demonstrated in 217 clinical studies and 180 journal publications (last update: January 2020).
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
L. reuteri Protectis® colonizes the entire gastrointestinal tract, prevents the growth of harmful microorganisms, activates the immune system and improves gastrointestinal function.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms.
It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
a) Protects the probiotic strain that surrounds it from
b) In human body
USE
GASTROINTESTINAL DISORDERS CAUSED BY ANTIBIOTICS
(diarrhoea, abdominal pain, flatulence)
PREVENTION & TREATMENT
DOSAGE (prevention)
1 tablet once daily between antibiotics + for 3 days afterwards
DOSAGE (treatment)
1 tablet once after each antibiotics administration + for 3 days after
_________________________________________________
FUNCTIONAL ABDOMINAL PAIN
(i.e. when there are no pathological causes)
TREATMENT
DOSAGE
1 tablet daily for at least 1 month
_________________________________________________
CHRONIC CONSTIPATION
(i.e. symptoms that persist for >2 weeks)
TREATMENT
DOSAGE
1 tablet once daily
_________________________________________________
SUDDEN GASTROINTESTINAL DISORDERS
(diarrhoea, vomiting, flatulence, etc.)
TREATMENT
DOSAGE
2 tablets once daily
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogiaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

BioGaia Protectis® Family
30x probiotic chewable tablets with lemon flavor
The world’s 1st probiotic in chewable tablets (2000)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For gasrtrointestinal health in children and adults
Gastrointestinal disorders caused by Antibiotics / Diarrhea / Constipation / Abdominal pain / Vomit
FOR USE BY
Children from 3 years and adults
_________________________________________________
_________________________________________________
BioGaia Protectis® probiotic chewable tablets are a simple, natural, safe and easy way to restore and maintain a healthy microbial balance in the gastrointestinal tract, as well as to improve digestive health and function.
BioGaia Protectis® drops contain L. reuteri Protectis® (L. reuteri DSM 17938), the patented probiotic bacterium of BioGaia Sweden. Originally isolated in breast milk, L. reuteri Protectis® is the world’s most studied probiotic in infants and children and one of the three most studied in adults. Its safety and efficacy have been demonstrated in 217 clinical studies and 180 journal publications (last update: January 2020).
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
L. reuteri Protectis® colonizes the entire gastrointestinal tract, prevents the growth of harmful microorganisms, activates the immune system and improves gastrointestinal function.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms.
It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
α) Protects the probiotic strain that surrounds it from
β) In human body
USE
GASTROINTESTINAL DISORDERS CAUSED BY ANTIBIOTICS
(diarrhoea, abdominal pain, flatulence)
PREVENTION & TREATMENT
DOSAGE (prevention)
1 tablet once daily between antibiotics + for 3 days afterwards
DOSAGE (treatment)
1 tablet once after each antibiotics administration + for 3 days after
_________________________________________________
FUNCTIONAL ABDOMINAL PAIN
(i.e. when there are no pathological causes)
TREATMENT
DOSAGE
1 tablet daily for at least 1 month
_________________________________________________
CHRONIC CONSTIPATION
(i.e. symptoms that persist for >2 weeks)
TREATMENT
DOSAGE
1 tablet once daily
_________________________________________________
SUDDEN GASTROINTESTINAL DISORDERS
(diarrhoea, vomiting, flatulence, etc.)
TREATMENT
DOSAGE
2 tablets once daily
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogiaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

Lacteeze® chewable tablets
100x chewable tablets with natural strawberry flavor
Natural lactase enzyme
For the breakdown of lactose before the consumption of solid and liquid foods and products containing lactose [e.g. yoghurt, cheese, biscuits etc. or fresh milk, powdered milk, condensed milk, breast milk, formula, chocolate milk, etc.]
FOR USE BY
Newborn infants (dissolved in milk, water, etc.), children and adults
_________________________________________________
_________________________________________________
LACTASE ENZYME
_________________________________________________
The enzyme lactase is naturally produced in the small intestine and it is responsible for breaking down lactose in foods containing it (dairy and products containing lactose) to enable its absorption by our body. As we grow older, the body’s production of lactase gradually decreases. The inhibition of its production also occurs after gastroenteritis, where the intestine is “injured” and it takes 1–2 weeks to return to its normal function in terms of enzyme production. Therefore, after gastroenteritis, Lacteeze® can help to continue consuming milk without problems.
Lacteeze® chewable tablets are a simple, natural, safe and easy way for our body to obtain the natural enzyme lactase, which is deficient when we are lactose intolerant. It has been estimated that in Greece ≈30% of children and ≈70% of adults suffer from lactose intolerance. Most often, symptoms are attributed to other causes, due to possible misdiagnosis. If you frequently suffer from bloating, gas or flatulence, abdominal pain or watery stools, you may have lactose intolerance. Lacteeze® restores nutritional completeness and taste satisfaction to those who avoid lactose-containing foods.
NOTE
Lacteeze® is not absorbed. Whatever amount does not interact with the lactose consumed through dairy products is eliminated from the organism naturally. Lacteeze® tablets should be taken every time you consume dairy products, just before consumption. Repeat the dose if dairy consumption continues after one hour. Adjust according to your needs. Take up to 3 times a day and no more than 12 tablets in total.
USE
BREASTFEEDING
Dissolve 1/4 of the tablet in a small amount of water or breast milk that you have extracted naturally or with a breast pump. Mix and give the mixture to the baby before you start breastfeeding
INFANT MILK
Dissolve 1/4 of the tablet in a small amount of water, milk that you have prepared. Mix and give the mixture to the baby before you start feeding
CHILDREN 2–3 YEARS
Do not give the tablet to children to swallow, there is a risk of choking. Dissolve 1 tablet in water or food just before eating dairy products
CHILDREN 4–12 YEARS
Chew 1 tablet just before consuming dairy products
ADULTS
Chew or swallow 1-3 tablets just before eating dairy products
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.lacteeze.eu
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

Lacteeze® drops
7 ml drops
Natural lactase enzyme
To break down the lactose contained in ≈30 litres of milk
FOR USE BY
Newborn infants, children and adults
_________________________________________________
_________________________________________________
LACTASE ENZYME
_________________________________________________
The enzyme lactase is naturally produced in the small intestine and it is responsible for breaking down lactose in foods containing it (dairy and products containing lactose) to enable its absorption by our body. As we grow older, the body’s production of lactase gradually decreases. The inhibition of its production also occurs after gastroenteritis, where the intestine is “injured” and it takes 1–2 weeks to return to its normal function in terms of enzyme production. Therefore, after gastroenteritis, Lacteeze® can help to continue consuming milk without problems.
Lacteeze® drops are a simple, natural, safe and easy way to break down lactose, which is found in dairy products that are in liquid form [e.g. fresh milk, milk powder, condensed milk, breast milk, infant milk powder (formula), chocolate milk and any other liquid milk product]. The drops must be added to the liquid containing lactose in order to break it down. It has been estimated that in Greece ≈30% of children and ≈70% of adults suffer from lactose intolerance. Most often, symptoms are attributed to other causes, due to possible misdiagnosis. If you frequently suffer from bloating, gas or flatulence, abdominal pain or watery stools, you may have lactose intolerance. Lacteeze® restores nutritional completeness and taste satisfaction to those who avoid lactose-containing foods.
NOTE
Lacteeze® is not absorbed. The amount that does not interact with the lactose consumed through dairy products is eliminated from the organism naturally. Milk, to which Lacteeze® has been added, has a slightly sweeter taste. This is natural, as it is due to the breakdown of lactose. The nutritional value of the milk is not affected.
USE
IN MILK
(fresh, pasteurized, chocolate, etc.)
Step 1
Add 5 drops of Lacteeze® per litre of milk
Step 2
Mix well and refrigerate the milk for 24 hours before consuming it (70–80% of the lactose is broken down). If you want to break down more lactose, you can increase the number of drops or leave the milk in the fridge for longer
BREASTFEEDING
Step 1
Extract 1 teaspoon of breast milk into a sterile container
Step 2
Add 4 drops of Lacteeze® to the container
Step 3
Give the mixture to the baby and continue breastfeeding normally (≈40% of the lactose is broken down). If you want to break down more lactose, you can increase the number of drops
IN BREAST MILK EXTRACTED WITH BREAST PUMP
Step 1
Add 4 drops of Lacteeze® per 50 ml of warm (not hot) breast milk extracted with a breast pump
Step 2
Shake well and wait 30 minutes before drinking. Do not refrigerate it. This will break down about 90% of the lactose. Alternatively, you can add 2 drops of Lacteeze® per 50 ml of warm breast milk. Shake well and wait 5 minutes before refrigerating it for 4 hours before consuming it. This will break down about 80% of the lactose.
IN INFANT FORMULA
Step 1
Add 4 drops of Lacteeze® per 50 ml of warm baby milk (not hot, about 30–40°C)
Step 2
Shake well and wait for 30 minutes before consuming. Do not refrigerate it. This will break down about 80% of the lactose. Alternatively, you can add 2 drops of Lacteeze® per 50 ml of warm baby milk. Shake well and wait 5 minutes before refrigerating it for 4 hours before consuming it. This will break down about 60% of the lactose
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.lacteeze.eu
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

BioGaia Protectis® ORS Child
7 sachets of probiotic hydration/electrolyte solution + zinc
The world’s 1st hydration solution with probiotic and zinc (2009)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For intestinal health in children and adults
Gastrenteritis / Dehydration / Diarrhea / Vomiting
FOR USE BY
Infants from birth, young children and adults
_________________________________________________
_________________________________________________
It contains electrolytes, probiotic L. reuteri Protectis® and zinc. Available in a package of 7 sachets of 5.5 g. The contents of each sachet are dissolved in 250 ml of cool water, kept 3 hours out of the refrigerator or 6 hours in the refrigerator, and provides the following:
* WHO: World Health Organization
* ESPGHAN: European Society of Paediatric Gastroenterology, Hepatology and Nutrition
* ESPID: European Society for Paediatric Infectious Diseases
_________________________________________________
BioGaia Protectis® ORS, probiotic oral hydration solution + zinc, is a simple, natural, safe and easy way to prevent and treat dehydration, e.g. caused by gastroenteritis, as well as to restore and maintain a healthy microbial balance in the gastrointestinal tract, in order to reduce the intensity and duration of symptoms associated with gastroenteritis.
BioGaia Protectis® ORS, probiotic oral hydration solution + zinc, is a simple, natural, safe and easy way to prevent and treat dehydration, e.g. caused by gastroenteritis, as well as to restore and maintain a healthy microbial balance in the gastrointestinal tract, in order to reduce the intensity and duration of symptoms associated with gastroenteritis.
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms, such as rotaviruses.
It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
a) Protects the probiotic strain that surrounds it from
b) In human body
Its action has been shown in large published clinical studies to provide:
_________________________________________________
+ ZINC
_________________________________________________
According to studies and observations by the WHO and UNICEF:
_________________________________________________
ELECTROLYTE COMPOSITION
_________________________________________________
The electrolyte composition of BioGaia Protectis® ORS is following the latest recommendations of WHO, UNICEF, ESPGHAN, ESPID.
According to the most recent studies and observations, the new formulation offers superior clinical benefits, and more specifically:
USE
ELECTROLYTIC DISORDERS AND DEHYDRATATION CAUSED BY GASTRENTERITIS (mild and moderate diarrhoea and/or vomiting), EPHIDROSIS, FEVER OR STRENUOUS EXERCISE, ETC.
PREVENTION & TREATMENT
DOSAGE
1–4 sachets daily, depending on age and needs.
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

Biogaia Protectis® Junior
30x probiotic chewable tablets with lemon flavor
The world’s 1st probiotic in chewable tablets (2000)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For gasrtrointestinal health in children and adults
Gastrointestinal disorders caused by Antibiotics / Diarrhea / Constipation / Abdominal pain / Vomiting
FOR USE BY
Children from 3 years and adults
_________________________________________________
_________________________________________________
BioGaia Protectis® probiotic chewable tablets are a simple, natural, safe and easy way to restore and maintain a healthy microbial balance in the gastrointestinal tract, as well as to improve digestive health and function.
BioGaia Protectis® drops contain L. reuteri Protectis® (L. reuteri DSM 17938), the patented probiotic bacterium of BioGaia Sweden. Originally isolated in breast milk, L. reuteri Protectis® is the world’s most studied probiotic in infants and children and one of the three most studied in adults. Its safety and efficacy have been demonstrated in 217 clinical studies and 180 journal publications (last update: January 2020).
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
L. reuteri Protectis® colonizes the entire gastrointestinal tract, prevents the growth of harmful microorganisms, activates the immune system and improves gastrointestinal function.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms.
It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
α)Protects the probiotic strain that surrounds it from
b) In human body
USE
GASTROINTESTINAL DISORDERS CAUSED BY ANTIBIOTICS
(diarrhoea, abdominal pain, flatulence)
PREVENTION & TREATMENT
DOSAGE (prevention)
1 tablet once daily between antibiotics + for 3 days afterwards
DOSAGE (treatment)
1 tablet once after each antibiotics administration + for 3 days after
_________________________________________________
FUNCTIONAL ABDOMINAL PAIN
(i.e. when there are no pathological causes)
TREATMENT
DOSAGE
1 tablet daily for at least 1 month
_________________________________________________
CHRONIC CONSTIPATION
(i.e. symptoms that persist for >2 weeks)
TREATMENT
DOSAGE
1 tablet once daily
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogiaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

BioGaia Protectis® Junior
10x probiotic chewable tablets with lemon flavor
The world’s 1st probiotic in chewable tablets (2000)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For gasrtrointestinal health in children and adults
Gastrointestinal disorders caused by Antibiotics / Diarrhoea / Constipation / Abdominal pain / Vomiting
FOR USE BY
Children from 3 years and adults
_________________________________________________
_________________________________________________
BioGaia Protectis® probiotic chewable tablets are a simple, natural, safe and easy way to restore and maintain a healthy microbial balance in the gastrointestinal tract, as well as to improve digestive health and function.
L.reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
L. reuteri Protectis® colonizes the entire gastrointestinal tract, prevents the growth of harmful microorganisms, activates the immune system and improves gastrointestinal function.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms.
It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
α) Protects the probiotic strain that surrounds it from
b) In human body
USE
GASTROINTESTINAL DISORDERS CAUSED BY ANTIBIOTICS
(diarrhoea, abdominal pain, flatulence)
PREVENTION & TREATMENT
DOSAGE (prevention)
1 tablet once daily between antibiotics + for 3 days afterwards
DOSAGE (treatment)
1 tablet once after each antibiotics administration + for 3 days after
_________________________________________________
FUNCTIONAL ABDOMINAL PAIN
(i.e. when there are no pathological causes)
TREATMENT
DOSAGE
1 tablet daily for at least 1 month
_________________________________________________
CHRONIC CONSTIPATION
(i.e. symptoms that persist for >2 weeks)
TREATMENT
DOSAGE
1 tablet once daily
_________________________________________________
SUDDEN GASTROINTESTINAL DISORDERS
(diarrhoea, vomiting, flatulence, etc.)
TREATMENT
DOSAGE
2 tablets once daily
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

BioGaia Protectis® + D3 Baby
5 ml of probiotic drops
The world’s 1st and No1 probiotic in drops (2004)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For infant colics and gastrointestinal health in infants and children
Gastrointestinal disorders caused by Antibiotics / Diarrhoea / Constipation / Abdominal pain / Vomit
With Vitamin D3: Proper immune function / Bone health / Prevention of rickets
FOR USE BY
Infants and toddlers
_________________________________________________
* European Paediatric Association (Union of National Paediatric Societies and Associations,
EPA/UNEPSA), World Gastroenterology Organisation (WGO), Asia-Pacific Pediatric Gastroenterologists, Latin American Pediatric Gastroenterologists.
** Independent meta-analysis published in the journal Pediatrics by Sung (2018).
*** Independent study published in JAMA Pediatrics by Indrio (2014).
_________________________________________________
BioGaia Protectis® drops contain L. reuteri Protectis® (L. reuteri DSM 17938), the patented probiotic bacterium of BioGaia Sweden. Originally isolated in breast milk, L. reuteri Protectis® is the world’s most studied probiotic in infants and children and one of the three most studied in adults. Its safety and efficacy have been demonstrated in 217 clinical studies and 180 journal publications (last update: January 2020).
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
L. reuteri Protectis® colonizes the entire gastrointestinal tract, prevents the growth of harmful microorganisms, activates the immune system and improves gastrointestinal function.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms.
+VITAMIN D
The recommended dosage of 5 drops per day provides 400 IU of vitamin D according to the European Food Safety Authority (EFSA) for infants and children. A daily intake of 400IU of vitamin D is recommended to meet the nutritional needs of vitamin D for proper bone development in infants and children and the prevention of rickets. Meeting these needs is often difficult due to the dependence of intake on each person’s diet, sun exposure, skin color and underlying diseases.
L. reuteri Protectis® has been shown to improve the body’s absorption of vitamin D and works synergistically to strengthen the immune system.
It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
a) Protects the probiotic strain that surrounds it from
b) In human body
USE – GASTROINTESTINAL DISORDERS
COLICS OF THE 1ST TRIMESTER
PREVENTION & TREATMENT
DOSAGE
5 drops once daily
_________________________________________________
GASTROINTESTINAL DISORDERS CAUSED BY TAKING ANTIBIOTICS IN INFANTS AND YOUNG CHILDREN
(diarrhoea, abdominal pain, flatulence)
PREVENTION & TREATMENT
DOSAGE (prevention)
5 drops once daily, between antibiotics + for 3 days afterwards
DOSAGE (treatment)
5 drops once 1 hour after each antibiotics administration + for 3 days after
_________________________________________________
GASTROINTESTINAL DISORDERS IN INFANTS DURING THE FIRST 3 MONTHS OF LIFE
(colic, constipation, reductions)
PREVENTION & TREATMENT
DOSAGE
5 drops once daily for the first 3 months
_________________________________________________
SUDDEN GASTROINTESTINAL DISORDERS
(diarrhoea, vomiting, flatulence, etc.)
TREATMENT
DOSAGE
10 drops once daily
USE – IMMUNE SYSTEM
HYPOVITAMINOSIS D
PREVENTION & TREATMENT
DOSAGE (prevention)
5 drops once daily
DOSAGE (treatment)
10 drops once daily
_________________________________________________
BOOSTING THE IMMUNE SYSTEM
PREVENTION
DOSAGE
5 drops once daily
_________________________________________________
WEAKENED IMMUNE SYSTEM
TREATMENT
DOSAGE
10 drops once daily
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma
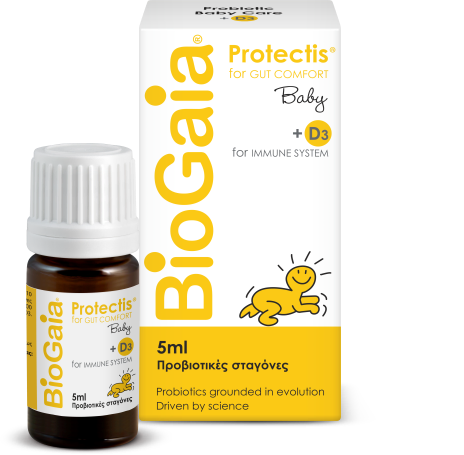
BioGaia Protectis® Baby
5 ml of probiotic drops
The world’s 1st and No1 probiotic in drops (2004)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For infant colics and gastrointestinal health in infants and children>
Gastrointestinal disorders caused by Antibiotics / Diarrhoea / Constipation / Abdominal pain / Vomit
FOR USE BY
Infants and toddlers
_________________________________________________
* European Paediatric Association (Union of National Paediatric Societies and Associations,
EPA/UNEPSA), World Gastroenterology Organisation (WGO), Asia-Pacific Pediatric Gastroenterologists, Latin American Pediatric Gastroenterologists.
** Independent meta-analysis published in the journal Pediatrics by Sung (2018).
*** Independent study published in JAMA Pediatrics by Indrio (2014).
_________________________________________________
BioGaia Protectis® drops contain L. reuteri Protectis® (L. reuteri DSM 17938), the patented probiotic bacterium of BioGaia Sweden. Originally isolated in breast milk, L. reuteri Protectis® is the world’s most studied probiotic in infants and children and one of the three most studied in adults. Its safety and efficacy have been demonstrated in 217 clinical studies and 180 journal publications (last update: January 2020).
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
L. reuteri Protectis® colonizes the entire gastrointestinal tract, prevents the growth of harmful microorganisms, activates the immune system and improves gastrointestinal function.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms.
It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
a) Protects the probiotic strain that surrounds it from
b) In human body
USE – GASTROINTESTINAL DISORDERS
COLICS OF THE 1ST TRIMESTER
PREVENTION & TREATMENT
DOSAGE
5 drops once daily
_________________________________________________
GASTROINTESTINAL DISORDERS CAUSED BY TAKING ANTIBIOTICS IN INFANTS AND YOUNG CHILDREN
(diarrhoea, abdominal pain, flatulence)
PREVENTION & TREATMENT
DOSAGE (prevention)
5 drops once daily, between antibiotics + for 3 days afterwards
DOSAGE (treatment)
5 drops once 1 hour after each antibiotics administration + for 3 days after
_________________________________________________
GASTROINTESTINAL DISORDERS IN INFANTS DURING THE FIRST 3 MONTHS OF LIFE
(colic, constipation, reductions)
PREVENTION & TREATMENT
DOSAGE
5 drops once daily for the first 3 months
_________________________________________________
SUDDEN GASTROINTESTINAL DISORDERS
(diarrhoea, vomiting, flatulence, etc.)
TREATMENT
DOSAGE
10 drops once daily
USE – IMMUNE SYSTEM
HYPOVITAMINOSIS D
PREVENTION & TREATMENT
DOSAGE (prevention)
5 drops once daily
DOSAGE (treatment)
10 drops once daily
_________________________________________________
BOOSTING THE IMMUNE SYSTEM
PREVENTION
DOSAGE
5 drops once daily
_________________________________________________
WEAKENED IMMUNE SYSTEM
TREATMENT
DOSAGE
10 drops once daily
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

Capricare® 1 Γάλα 2ης βρεφικής ηλικίας
400gr
The Original (1988) and No1 goat milk infant formula worldwide
Based on WHOLE goat milk
The only goat milk for infants evaluated by EFSA
FOR USE BY
Infants from 6 to 12 months
_________________________________________________
_________________________________________________
Milk powder for the 2nd infant age of infants from the 6th to the 12th month and for the weaning period. It is prepared with whole goat milk as raw material instead of mixing ingredients, resulting in the mildest possible processing.
It complies with the European Infant Formula Directive and it is the only one clinically evaluated by the European Food Safety Agency (EFSA).
It is produced by the world leader in research, development and production of whole goat milk for infants, Dairy Goat Co-operative (DGC) New Zealand.
DGC’s unique and vertically integrated production process (breeding – milking – milk powder production – manufacturing of packaging materials – canning) ensures:
FASTEST PROCESS POSSIBLE
From milking the goats to milk powder within couple of days. It ensures the freshness and natural properties of whole goat milk.
LESS HARM TO THE RAW MATERIAL
The mildest heat treatment, with the least possible “disturbance” of the proteins and the lowest percentage of glycosylated compounds in the final product.
REDUCED RISK OF CONTAMINATION
No long-term storage of ingredients, large number of suppliers, transport and processing in other factories or countries.
QUALITY ASSURANCE
30 years of expertise. 100% focus on exclusive production of infant milk from whole goat milk. Total control of raw materials, packaging materials and all stages of production, ensuring the quality of the final product.
RESULT
The benefits of the unique natural properties of whole goat milk are retained in the final product:
_________________________________________________
DAIRY GOAT CO-OPERATIVE (DGC)
_________________________________________________
Dairy Goat Co-operative (DGC) Ltd., Nea Zealand, is the world leader in infant milk from goat milk. DGC is the inventor of infant milk from goat milk (1988). Throughout these 30 years, DGC has managed to keep a continuous evolution and to develop a highly sophisticated model of expertise around infant milk based on goat milk, that lead to the change of the European legislation for infant milks in 2012. DGC is a highly integrated co-operation, where every step in infant milk production, from farming and milking to infant milk in the can, is handled, controlled and completed in whole by DGC. The final product is produced and packaged in the world’s most sophisticated production facility for infant milk, designed to produce exclusively infant milk from whole goat milk.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.capricare.gr
www.facebook.com/Capricare.Greece
www.instagram.com/capricaregreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

Capricare® 2 Γάλα 2ης βρεφικής ηλικίας
400gr
The Original (1988) and No1 goat milk infant formula worldwide
Based on WHOLE goat milk
The only goat milk for infants evaluated by EFSA
FOR USE BY
Infants from 6 to 12 months
_________________________________________________
_________________________________________________
Milk powder for the 2nd infant age of infants from the 6th to the 12th month and for the weaning period. It is prepared with whole goat milk as raw material instead of mixing ingredients, resulting in the mildest possible processing.
It complies with the European Infant Formula Directive and it is the only one clinically evaluated by the European Food Safety Agency (EFSA).
It is produced by the world leader in research, development and production of whole goat milk for infants, Dairy Goat Co-operative (DGC) New Zealand.
DGC’s unique and vertically integrated production process (breeding – milking – milk powder production – manufacturing of packaging materials – canning) ensures:
FASTEST PROCESS POSSIBLE
From milking the goats to milk powder within couple of days. It ensures the freshness and natural properties of whole goat milk.
LESS HARM TO THE RAW MATERIAL
The mildest heat treatment, with the least possible “disturbance” of the proteins and the lowest percentage of glycosylated compounds in the final product.
REDUCED RISK OF CONTAMINATION
No long-term storage of ingredients, large number of suppliers, transport and processing in other factories or countries.
QUALITY ASSURANCE
30 years of expertise. 100% focus on exclusive production of infant milk from whole goat milk. Total control of raw materials, packaging materials and all stages of production, ensuring the quality of the final product.
RESULT
The benefits of the unique natural properties of whole goat milk are retained in the final product:
_________________________________________________
DAIRY GOAT CO-OPERATIVE (DGC)
_________________________________________________
Dairy Goat Co-operative (DGC) Ltd., Nea Zealand, is the world leader in infant milk from goat milk. DGC is the inventor of infant milk from goat milk (1988). Throughout these 30 years, DGC has managed to keep a continuous evolution and to develop a highly sophisticated model of expertise around infant milk based on goat milk, that lead to the change of the European legislation for infant milks in 2012. DGC is a highly integrated co-operation, where every step in infant milk production, from farming and milking to infant milk in the can, is handled, controlled and completed in whole by DGC. The final product is produced and packaged in the world’s most sophisticated production facility for infant milk, designed to produce exclusively infant milk from whole goat milk.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.capricare.gr
www.facebook.com/Capricare.Greece
www.instagram.com/capricaregreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

Lacteeze® chewable tablets
10x chewable tablets with natural strawberry flavor
Natural lactase enzyme
For the breakdown of lactose before the consumption of solid and liquid foods and products containing lactose [e.g. yoghurt, cheese, biscuits etc. or fresh milk, powdered milk, condensed milk, breast milk, formula, chocolate milk, etc.]
FOR USE BY
Newborn infants (dissolved in milk, water, etc.), children and adults
_________________________________________________
_________________________________________________
LACTASE ENZYME
_________________________________________________
The enzyme lactase is naturally produced in the small intestine and it is responsible for breaking down lactose in foods containing it (dairy and products containing lactose) to enable its absorption by our body. As we grow older, the body’s production of lactase gradually decreases. The inhibition of its production also occurs after gastroenteritis, where the intestine is “injured” and it takes 1–2 weeks to return to its normal function in terms of enzyme production. Therefore, after gastroenteritis, Lacteeze® can help to continue consuming milk without problems.
Lacteeze® chewable tablets are a simple, natural, safe and easy way for our body to obtain the natural enzyme lactase, which is deficient when we are lactose intolerant. It has been estimated that in Greece ≈30% of children and ≈70% of adults suffer from lactose intolerance. Most often, symptoms are attributed to other causes, due to possible misdiagnosis. If you frequently suffer from bloating, gas or flatulence, abdominal pain or watery stools, you may have lactose intolerance. Lacteeze® restores nutritional completeness and taste satisfaction to those who avoid lactose-containing foods.
NOTE
Lacteeze® is not absorbed. Whatever amount does not interact with the lactose consumed through dairy products is eliminated from the organism naturally. Lacteeze® tablets should be taken every time you consume dairy products, just before consumption. Repeat the dose if dairy consumption continues after one hour. Adjust according to your needs. Take up to 3 times a day and no more than 12 tablets in total.
USE
BREASTFEEDING
Dissolve 1/4 of the tablet in a small amount of water or breast milk that you have extracted naturally or with a breast pump. Mix and give the mixture to the baby before you start breastfeeding
INFANT MILK
Dissolve 1/4 of the tablet in a small amount of water, milk that you have prepared. Mix and give the mixture to the baby before you start feeding
CHILDREN 2–3 YEARS
Do not give the tablet to children to swallow, there is a risk of choking. Dissolve 1 tablet in water or food just before eating dairy products
CHILDREN 4–12 YEARS
Chew 1 tablet just before consuming dairy products
ADULTS
Chew or swallow 1-3 tablets just before eating dairy products
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.lacteeze.eu
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma

