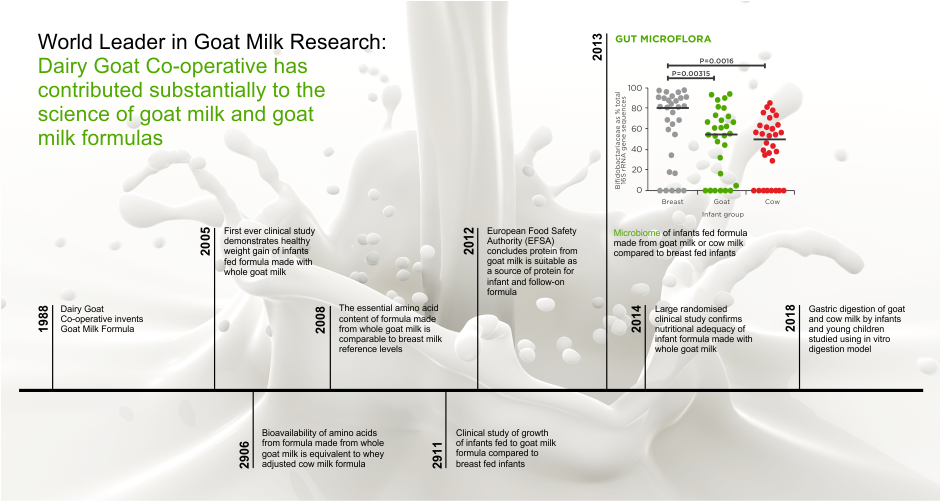
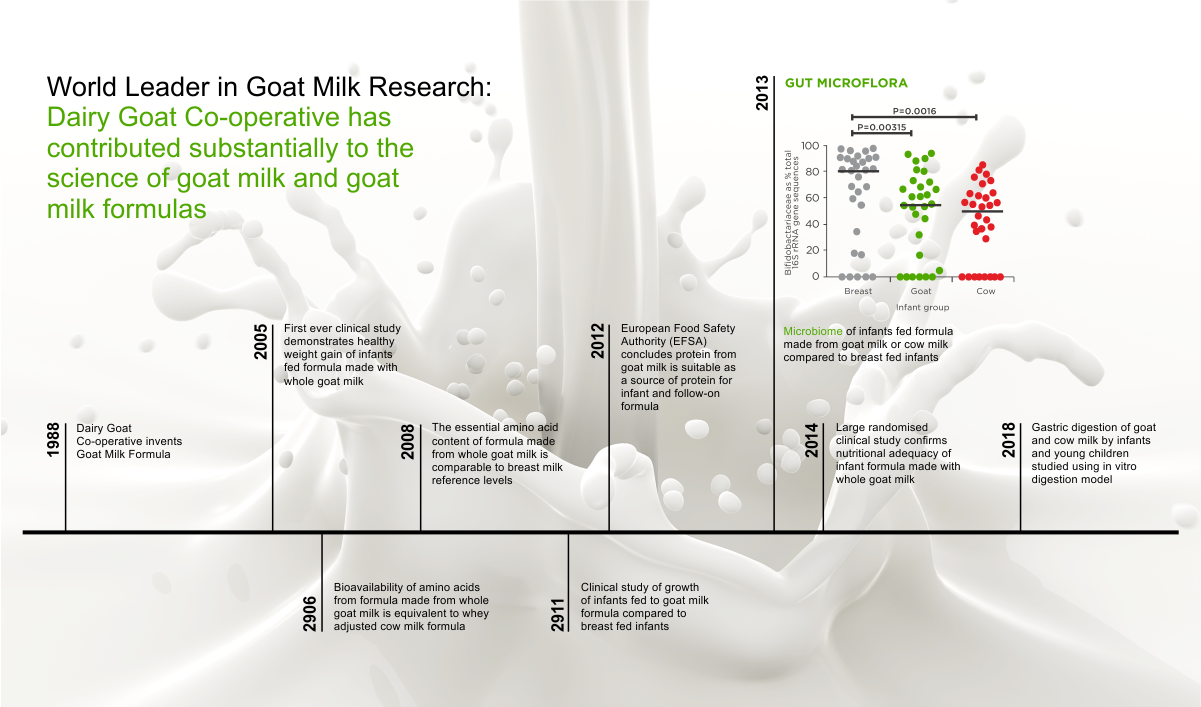
7 sachets of probiotic hydration/electrolyte solution + zinc
The world’s 1st hydration solution with probiotic and zinc (2009)
Unique probiotic, with probiotic and prebiotic properties at the same time…by its nature!
For intestinal health in children and adults
Gastrenteritis / Dehydration / Diarrhea / Vomiting
FOR USE BY
Infants from birth, young children and adults
_________________________________________________
- Suitable from birth
- It contains the world’s most studied probiotic strain in infants and children, one of the three most studied in adults + zinc (antimicrobial activity)
- Human strain, isolated in breast milk
- Natural colonizer of our gastrointestinal tract from birth
- With unique, parallel, natural, prebiotic action, due to its natural structure
- Clinically proven to colonize the entire gastrointestinal tract
- Clinically proven per clinical benefit and age group
- Clinically proven, unique, natural, anti-pathogenic activity
- Safe for infants, children and adults, during pregnancy and breastfeeding
- Patented
- It contains no lactose, gluten, milk protein.
_________________________________________________
It contains electrolytes, probiotic L. reuteri Protectis® and zinc. Available in a package of 7 sachets of 5.5 g. The contents of each sachet are dissolved in 250 ml of cool water, kept 3 hours out of the refrigerator or 6 hours in the refrigerator, and provides the following:
- Electrolytes and glucose
[composition based on the most recent recommendations (WHO, UNICEF, ESPGHAN, ESPID)]*
- Probiotic strain L. reuteri Protectis®
[based on the official guidelines of ESPGHAN (2014) for the treatment of acute gastroenteritis in children]
- Zinc
(based on WHO, UNICEF recommendations)
- Low osmolality (220 mOsm/kg)
(based on WHO, ESPGHAN recommendations)
* WHO: World Health Organization
* ESPGHAN: European Society of Paediatric Gastroenterology, Hepatology and Nutrition
* ESPID: European Society for Paediatric Infectious Diseases
_________________________________________________
BioGaia Protectis® ORS, probiotic oral hydration solution + zinc, is a simple, natural, safe and easy way to prevent and treat dehydration, e.g. caused by gastroenteritis, as well as to restore and maintain a healthy microbial balance in the gastrointestinal tract, in order to reduce the intensity and duration of symptoms associated with gastroenteritis.
BioGaia Protectis® ORS, probiotic oral hydration solution + zinc, is a simple, natural, safe and easy way to prevent and treat dehydration, e.g. caused by gastroenteritis, as well as to restore and maintain a healthy microbial balance in the gastrointestinal tract, in order to reduce the intensity and duration of symptoms associated with gastroenteritis.
L. reuteri Protectis® fully meets the requirements of WHO for probiotics. In an in vitro study comparing the viability of 35 different probiotic strains in a simulated human gastrointestinal tract over a period of five days, L. reuteri Protectis® was shown to be one of the only three surviving strains. This was also confirmed in vivo, when ingestion from healthy humans led to significant colonisation of the stomach, duodenum and ileum.
In addition, it is the only probiotic that produces the antimicrobial substance rheuterine against pathogenic microorganisms, such as rotaviruses.
It contains the unique probiotic strain Limosilactobacillus reuteri Protectis®
(former name Lactobacillus reuteri Protectis®).
The unique natural substance Limosi –a high molecular weight exopolysaccharide biofilm (EPS)– surrounding the probiotic strain L. reuteri Protectis® also acts as a prebiotic for the symbiotic flora.
This biofilm:
a) Protects the probiotic strain that surrounds it from
- other micro-organisms
- antibiotics
- toxic compounds
- osmotic stress
b) In human body
- It inhibits the adhesion of pathogens to the intestinal epithelium.
- It supports the symbiotic microflora, acting as a prebiotic (food).
- It regulates the immune system by activating T-regulatory cells and increasing anti-inflammatory cytokines.
Its action has been shown in large published clinical studies to provide:
- 74% reduction in episodes of watery diarrhea from day 2 in hospitalized children
- 50% reduction in episodes of vomiting from day 2 in neonates and infants
- 45% additional reduction in the duration of diarrhoea with early intake of the probiotic
_________________________________________________
+ ZINC
_________________________________________________
According to studies and observations by the WHO and UNICEF:
- Zinc can reduce the duration and intensity of diarrhea.
- It can reduce the vomiting episodes.
- It does not affect the action of electrolytes.
_________________________________________________
ELECTROLYTE COMPOSITION
_________________________________________________
The electrolyte composition of BioGaia Protectis® ORS is following the latest recommendations of WHO, UNICEF, ESPGHAN, ESPID.
According to the most recent studies and observations, the new formulation offers superior clinical benefits, and more specifically:
- It reduces the number of defecations by 20%.
- It reduces vomiting episodes by 30%.
- It reduces the need for emergency intravenous treatment by 33%.
- It does not increase the possibility of hyponatraemia.
USE
ELECTROLYTIC DISORDERS AND DEHYDRATATION CAUSED BY GASTRENTERITIS (mild and moderate diarrhoea and/or vomiting), EPHIDROSIS, FEVER OR STRENUOUS EXERCISE, ETC.
PREVENTION & TREATMENT
DOSAGE
1–4 sachets daily, depending on age and needs.
_________________________________________________
BioGaia AB
_________________________________________________
BioGaia AB is a Swedish biotechnology company and a global leader in probiotics, with a strong innovation profile, experience and expertise of over 30 years. To date, BioGaia AB holds more than 550 patents, a commercial presence in more than 100 countries and a physical presence in Europe, USA, China and Japan. BioGaia’s vision is to improve people’s health by offering probiotic products that are clinically documented and proven for safety and efficacy by clinical benefit and age group. Until now (2/2020) BioGaia AB has completed more than 217 clinical studies in infants, children and adults and has demonstrated the safety and efficacy of its probiotic products in various disorders.
_________________________________________________
PRODUCT NOTIFIED TO THE NATIONAL ORGANIZATION FOR MEDICINES (ΕΟΦ)
_________________________________________________
www.biogaia.gr
www.facebook.com/Biogaia.Greece
www.instagram.com/biogaiagreece
www.cube-pharmaceuticals.gr
www.facebook.com/Cube.Pharma
www.instagram.com/cubepharma